Poliomyelitis
Clinical manifestation:
- Few suffer from minor illness, very few suffer from meningitis and less than 1% suffer from major paralytic disease
1. Asymptomatic illness:
- In most of the infection is asymptomatic and self-limiting
- 99% cases
2. Abortive poliomyelitis:
- Non-specific symptoms such as headache, fever, sore throat, loss of appetite
- Disease last for 5 days
3. Non paralytic poliomyelitis
- Very few patients suffer from non-paralytic poliomyelitis
- Stiffness of neck
- Pain in back and neck
- Disease last for 2-10 days
4. Paralytic Poliomyelitis:
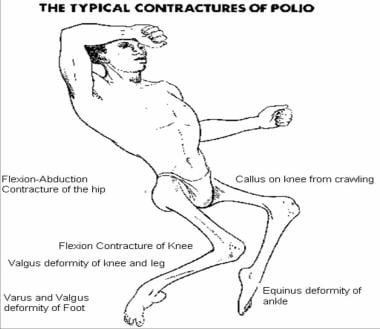
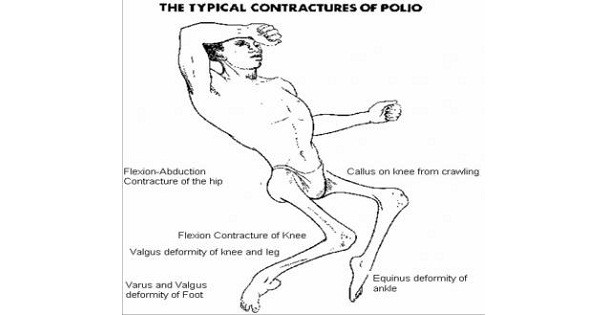
- Less than 1% patients suffer from major paralytic poliomyelitis
- Paralysis in polio is asymmetric, descending, non-progressive and LMN type.
- It damages the motor nerves causing oedema and muscle paralysis
- Malaise
- Anorexia
- Nausea and vomiting
- Sore throat
- Constipation
- Abdominal pain
- Headache and fever
- Flaccid paralysis: motor neuron damage
- Lower motor neuron lesion of the anterior horn cells of the spinal cord and affects the muscles of the legs, arms and/ or trunk
- Bulbar paralysis: respiratory paralysis
5. Post-poliomyelitis muscle atrophy:
- Muscle wasting
- Loss of neuromuscular function
- Physically Disabled
6. Death is rare. And if occur it is due to respiratory paralysi
Lab diagnosis:
- Specimen: nasal secretion, faecal samples, throat swab, CSF
- Electron Microscopy: virus detection
- Virus isolation from stool:
- Culture on monkey kidney cell line, Human amnion, HeLa, Hep-2, Buffalo green monkey (BGM), MRC-5 cell line
- Antibody detection: ELISA, complement fixation test
- Antigen detection: neutralization test
- Molecular diagnosis: PCR
Treatment: no antiviral drugs
Prevention and control:
1. Vaccination
i. Inactivated Polio Vaccine (Salk type – killed):
- Prepared by Jonas Salk in 1956
- Also known as Inactivated poliovirus vaccine (IPV)
- Prepared by formalin inactivation of poliovirus
- It is injected deep subcutaneous or intramuscular
- Given to child at age of 2 months, 4 months, at school entry age
- Effective against all serotype of poliovirus
- Produces circulatory antibody
- Does NOT require stringent refrigeration
- NOT effective in an epidemic
ii. Oral Polio Vaccine (Sabin type – live):
- Developed by Albert Sabin in 1962
- Contains live attenuated strain of all serotypes of poliovirus
The vaccine contains :-
- Over 300,000 TCID 50 of type 1 poliovirus
- Over 100,000 TCID 50 of type 2 poliovirus
- Over 300,000 TCID 50 of type 3 poliovirus
- Dose 2 drop (0.1 ml)
- Schedule in National Immunization Programme of India.( 5 doses)
Dose and Age
- OPV-0 (Zero dose)- At birth
- OPV-1 -6 weeks
- OPV-2 -10 weeks
- OPV-3 – 14 weeks
- OPV-B (Booster dose) – 16-24 months
- On administration, the virus multiplies in the intestine
- Induces intestinal and systemic immunity
- Intestinal immunity prevents infection of intestine by Poliovirus
- Vaccine virus is excreted in the feces and can infect the unimmunized and induce immunity
- Thus widespread herd immunity results, even if only approximately 66% of the community is immunized (100% coverage is not required).
- It is administered Orally at 2 months of age simultaneously with first DPT
- It is recommended for all children below 5 years
- In endemic countries monovalent oral poliovirus type I vaccine (MOPvI) is introduced to eliminate the last reservoir of poliovirus
- Colostrum produced in the first three days after child-birth contains secretory IgA antibody which might interfere with the production of immune response to OPV
- Nevertheless, several studies show that among breastfed infants who are fed OPV in the first three days of life, 20-40 percent develop serum antibodies and 30-60 percent excrete vaccine virus.
- Molar concentration of certain salts MgC12, Na2So4 protect polio virus from heat inactivation
- As it prevents heat inactivation mgCl2 can be added to polio vaccine so that it can be stored at a higher temperature.
Complications of OPV
- Being living viruses, the vaccine viruses, particularly type 3 do mutate
- In the course of their multiplication in vaccinated children, and rare cases of vaccine associated paralytic polio have occured in –
- Recipients of the vaccine
- Thier contacts
Contraindiations of OPV
- Immunocompromized individuals
- Patients suffering from leukaemias & malignancy or AIDS.
- Persons receiving corticosteroids.
- In pregnancy.
2. Proper sanitation
3. Safe drinking water
Vaccine derived polio virus (VDPV)
Types of VDPV:
- c-VDPV: Person-to-person transmission in community
- i-VDPV: Isolates from immunodeficient persons
- a-VDPV: Ambiguous from health person or sewage isolates
Diagnosis:
- VDPV is diagnosed by Real-time Reverse transcription-PCR nucleic acid amplification
Key risk factors for cVDPV emergence
| Development of immunity gaps (due to low OPV coverage) |
Low routine immunization coverage with trivalent OPV |
| Prior elimination of WPV types | Insensitive AFP surveillance |
Clinical manifestation:
- Few suffer from minor illness, very few suffer from meningitis and less than 1% suffer from major paralytic disease
1. Asymptomatic illness:
- In most of the infection is asymptomatic and self-limiting
- 99% cases
2. Abortive poliomyelitis:
3. Non paralytic poliomyelitis
4. Paralytic Poliomyelitis:
- Less than 1% patients suffer from major paralytic poliomyelitis
- Paralysis in polio is asymmetric, descending, non-progressive and LMN type.
- Flaccid paralysis: motor neuron damage
- Lower motor neuron lesion of the anterior horn cells of the spinal cord and affects the muscles of the legs, arms and/ or trunk
- Bulbar paralysis: respiratory paralysis
5. Post-poliomyelitis muscle atrophy
6. Death is rare. And if occur it is due to respiratory paralysis
Lab diagnosis:
- Specimen: nasal secretion, faecal samples, throat swab, CSF
- Electron Microscopy: virus detection
- Virus isolation from stool
Prevention and control:
1. Vaccination
i. Inactivated Polio Vaccine (Salk type – killed):
- Effective against all serotype of poliovirus
- Produces circulatory antibody
- Does NOT require stringent refrigeration
- NOT effective in an epidemic
ii. Oral Polio Vaccine (Sabin type – live):
- Developed by Albert Sabin in 1962
The vaccine contains :-
- Over 300,000 TCID 50 of type 1 poliovirus
- Over 100,000 TCID 50 of type 2 poliovirus
- Over 300,000 TCID 50 of type 3 poliovirus
- Dose 2 drop (0.1 ml)
- Schedule in National Immunization Programme of India.( 5 doses)
Dose Age
- OPV-0 (Zero dose)- At birth
- OPV-1 -6 weeks
- OPV-2 -10 weeks
- OPV-3 – 14 weeks
- OPV-B (Booster dose) – 16-24 months
- On administration, the virus multiplies in the intestine
- Induces intestinal and systemic immunity
- Intestinal immunity prevents infection of intestine by Poliovirus
- Vaccine virus is excreted in the feces and can infect the unimmunized and induce immunity
- Thus widespread herd immunity results, even if only approximately 66% of the community is immunized (100% coverage is not required).
- In endemic countries monovalent oral poliovirus type I vaccine (MOPvI) is introduced to eliminate the last reservoir of poliovirus
- Nevertheless, several studies show that among breastfed infants who are fed OPV in the first three days of life, 20-40 percent develop serum antibodies and 30-60 percent excrete vaccine virus.
- Molar concentration of certain salts MgC12, Na2So4 protect polio virus from heat inactivation
- As it prevents heat inactivation mgCl2 can be added to polio vaccine so that it can be stored at a higher temperature.
- Complications of OPV
- In the course of their multiplication in vaccinated children, and rare cases of vaccine associated paralytic polio have occurred in –
- Recipients of the vaccine
- Thier contacts
- Vaccine derived polio virus (VDPV)
Types of VDPV:
- c-VDPV: Person-to-person transmission in community
- i-VDPV: Isolates from immunodeficient persons
- a-VDPV: Ambiguous from health person or sewage isolates
IM injections and increased muscular activity lead to increased paralysis